The Berntsson lab at the department of Medical Biochemistry and Biophysics at Umeå University, Sweden, is recruiting one postdoctoral fellow (funded by the Kempe Foundation) to study the structural and functional aspects of Type 4 Secretion Systems. These large protein complexes are responsible for horizontal gene transfer between bacteria. As such, they facilitate the spread of antibiotic resistance genes and virulence factors between bacteria, both intra- and interspecies.
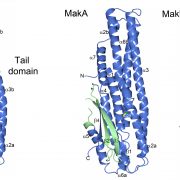
The overall goal of this project is to study the assembly and structure of the membrane channel of Gram-positive Type 4 Secretion Systems, with the aim of deducing their structure via cryo-EM. Initial work on this project has already been done, with a focus on the individual components of the channel (both unpublished data and Jäger et al, 2020, bioRxiv doi: 10.1101/2020.10.30.342212), which we will now build upon. Here at Umeå University we have access to excellent facilities to perform integrated structural biology projects, including a state-of-the-art cryo-EM facility (https://tinyurl.com/b2szms7z).
Qualifications
Applicants must possess a PhD, or another diploma deemed equivalent to a PhD, within molecular biology, biochemistry, structural biology or a related field. Furthermore, the applicant must have practical experience and expertise of cloning, protein production and purification in/from bacteria. Previous experience with cryo-EM (single particle analysis or electron tomography) is highly desirable. Previous experience of working with membrane proteins is an advantage, as is experience with Gram-positive bacteria, like E. faecalis. The applicant must have a very good level of English, both written and spoken. To fit into our team, applicants are expected to be good team players, but they should also be able to work independently.
Application
The application should consist of the following:
- A motivation letter (max 1 A4), where you highlight why you want to join the lab and study T4SSs. This letter must also include your contact information.
- The Curriculum Vitae of the applicant, including a list of published peer-reviewed articles.
- Copies of the PhD diploma (or equivalent).
- Contact details for three references, of which one should be your PhD supervisor.
The duration of the fellowship, which is a tax-free stipend, is 2 years. Your application should be written in English and prepared as a single package in PDF format, to be submitted to ronnie.berntsson@umu.se. Make sure to use the subject line: “postdoc application 2021-05” in the application email. Submit the application on May 21st, 2021 at the latest. The top ranked candidates will be contacted within two weeks from the closing date for an interview. Starting date will be according to agreement (and of course what the current pandemic situation allows). For more information about the research or other details, please contact Dr. Ronnie Berntsson.
Email: ronnie.berntsson@umu.se
Webpage: https://www.biostruct.umu.se/principal-investigators/ronnie-berntsson/